Corresponding author: Jacob Guggenheim (jguggenh@mit.edu)
Introduction
Cellular heterogeneity plays a critical role in processes like embryonic development, drug-resistance, and immune response. The ability to select and manipulate individual cells greatly increases the ability to study heterogeneity within cultures. As such, a number of different single cell sorting techniques have been developed. While filling their respective niches, there does not currently exist a system capable of sorting individual cells based upon an arbitrary set of morphological or otherwise observable features while providing good enough throughput to deal with working within biologically relevant timescales. To address this need, an integrated system for the selection and manipulation of single cells in 2D culture was developed by automating both the selection of cells using computer vision and manipulation of cells using micro-capillaries.
Design
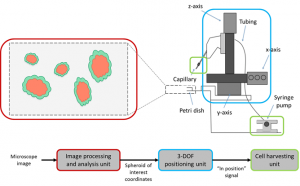
Our system consists of three major units: an image processing and analysis unit, a 3-axis positioning unit, and a cell harvesting unit. The system can be replicated for relatively low-cost from off-the-shelf components as a custom build to fit almost any existing microscope. The image processing and analysis unit takes as input a series of real-time images from the microscope and analyzes them for cells of interest. When a cell of interest is located, the image processing unit directs the 3-axis positioning unit to move a micropipette into place. Finally, the cell harvesting unit takes up a single cell while minimally affecting the neighboring spheroids and holds it until all the collected cell are dispensed.
Research
Organoids are cell aggregates that arise in vitro with three-dimensional structure, which more closely mimics the in vivo tissue than two-dimensional models. Given the proper environment and growth factors, pluripotent stem cells (PSCs) will differentiate into organoids of many tissues including brain, liver, kidney, stomach, and intestine. A mechanistic understanding of early organoid formation is essential for transitioning these technologies into robust, high-throughput methods that are suitable for commercial and therapeutic applications.
Spheroids are early stage cell clusters that may successfully mature into intestinal organoids. The spheroids that bud off the definitive endoderm are highly heterogeneous, varying by number of cells per spheroid, presence of an inner mass and inner mass diameter, total diameter, and cell type distribution.
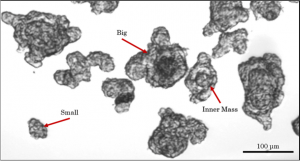
Only ~12% of spheroids that bud of eventually mature into intestinal organoids. Given the high morphological heterogeneity of the spheroids, it was hypothesized that by sorting for particular morphological features the successful maturation percentage of the spheroids into intestinal organoids could be increased, thus making the protocol into a robust, high throughput method for use in drug delivery and development.
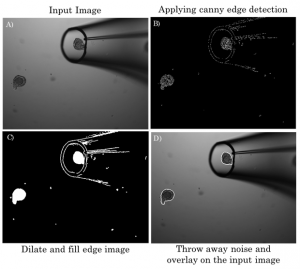
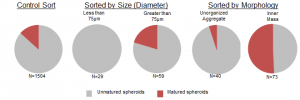
The first step toward sorting required that the spheroids be segmented from the background of the microscope images. Under brightfield illumination, the suspended spheroids stand off from the background. To detect this, the Canny Edge Detection Algorithm was applied. This provided clusters of edges that were connected by morphologically dilating and filling the image. The connected regions were then morphologically opened to remove thin segments, and the entire image was de-noised by removing any connected regions with a pixel area smaller than a threshold. Finally, any connected region touching the border of the image was also removed as it complicated classification. A condensed version of the algorithm is shown above. The spheroids are then classified by diameter (using a 75um threshold) and separately by presence of an inner mass. Spheroids that are greater than 75μm were mature 1.5-fold more often into organoids than the general population while spheroids that displayed an inner mass mature 3.8 fold more often.
Publications
N Arora*, J Imran Alsous*, J W Guggenheim*, M Mak, J Munera, J M Wells, R D Kamm, H H Asada, S Y Shvartsman, L G Griffith, “A Process Engineering Approach to Increase Organoid Yield,” Development, 2017
* Equal contribution